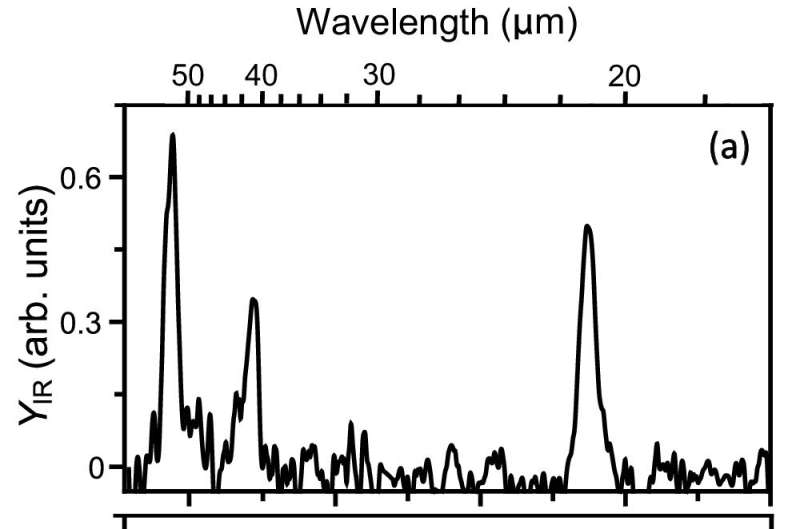
Infrared spectrum of neutral S8. Credit: Nature Communications (2024). DOI: 10.1038/s41467-024-50303-2
For the first time, a team of scientists from HFML-FELIX at Radboud University has unveiled the cosmic fingerprints of sulfur rings. These results, published in Nature Communications, may shed new light on the way sulfur was transported from dark interstellar clouds (where stars are formed) to young planetary systems and planets like Earth and Venus, and offer ways to search for cosmic sulfur using the James Webb Space Telescope (JWST).
Sulfur is essential to all living things and is one of six chemical elements that make up DNA. But its path from interstellar clouds to planets and their atmospheres is not well known. Sulfur is found in diffuse gas clouds in our galaxy. However, when these gas clouds contract to form new stars and planets, most sulfur escapes human detection.
Beamline scientist Piero Ferrari says, "So far, only 1% of the cosmic reservoir of sulfur had been accounted for in space, for example in sulfur dioxide or hydrogen sulfide. It remains unclear how and in what form sulfur is delivered to young planet-forming disks, such as Earth or Venus."
To solve the missing sulfur problem, astrochemists have speculated that atomic sulfur can be locked into a large all-sulfur ring molecule. Using models to simulate chemical reactions in dark interstellar clouds (where stars and planets are formed), they found that chains and rings of sulfur can form, especially the ring-shaped S8.
Recently, this was lent credibility, when sulfur molecules containing up to four sulfur atoms were discovered in the Rosetta comet and in an asteroid.
However, their stability is not well understood, leaving open the question of how such rings and chains can survive during planet formation. Furthermore, their presence in dark clouds could not be established because this requires knowing their infrared spectra.
Using the free-electron laser at HFML-FELIX, the team has for the first time recorded the infrared spectral signature of the most stable all-sulfur molecule, octasulfur or S8, and of several smaller sulfur molecules. This opens up the possibility of searching for these molecules in interstellar dark clouds using the powerful James Webb Space Telescope.
Ferrari says, "However, preliminary estimates indicate that detecting S8 with JWST will still be challenging."
The team was also able to find out how S8 breaks up, i.e. what the fragments are, and how much energy that takes. The new data show that S8 is more fragile than previously assumed in models, which means its survival may be jeopardized by high-energy photons or cosmic particles.
The data are of great importance for astrochemical models, which treat the chemical pathways linking the formation of molecules in interstellar environments and their presence in planetary atmospheres and interiors.
Ferrari adds, "By knowing the cosmic fingerprints of the mysterious but crucial element of sulfur, we can focus our search for ways in which sulfur reaches new planets in our galaxy. This allows us a better look at how life is formed."
More information: Piero Ferrari et al, Laboratory infrared spectra and fragmentation chemistry of sulfur allotropes, Nature Communications (2024). DOI: 10.1038/s41467-024-50303-2
Journal information: Nature Communications
Provided by Radboud University