This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Discovery of a tRNA modification enzyme that also acts on nucleosides
The genetic information on DNA is transcribed into messenger RNA (mRNA) and translated to the amino acid sequence by transfer RNA (tRNA) on the ribosome. Modified nucleosides within RNA are involved in maintaining and regulating the protein synthesis system.
Archaeosine is a modified nucleoside found only in the tRNAs from archaea, the so-called third domain of life, and contributes to the maintenance of the L-shaped tRNA three-dimensional structure.
The synthesis of archaeosine involves multiple steps, with the first step introducing a preQ0 base into tRNA via ArcTGT. In the second step, ArcS transfers an amino acid, lysine, to the preQ0 base in tRNA and synthesizes preQ0-Lys as an intermediate. The resultant preQ0-Lys in tRNA is then converted into archaeosine by RaSEA, the third-step enzyme.
This synthesis pathway of archaeosine was elucidated in 2019 through a collaborative study by Ehime University and Gifu University published in Nature Chemical Biology. However, the substrate specificity of the second-step enzyme ArcS was previously unknown.
To address this issue, a research group led by Professor Hiroyuki Hori, Lecturer Dr. Ryota Yamagami, and graduate students Shu Fujita, Yuzuru Sugio, and Dr. Takuya Kawamura (currently at Thomas Jefferson University, U.S.) at the Graduate School of Science and Engineering, Ehime University, in collaboration with Professors Takashi Yokogawa and Natsuhisa Oka from Gifu University and Associate Professor Akira Hirata from Tokushima University, conducted biochemical analyses.
The results of the study are published in the Journal of Biological Chemistry.
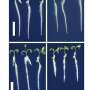
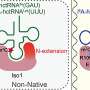
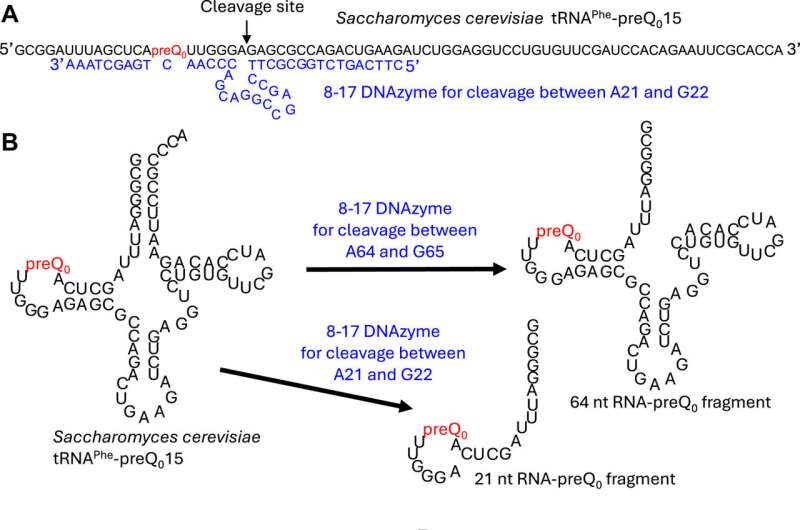
Most RNA modification enzymes recognize the three-dimensional structure around the target site of RNA and only rarely the RNA sequence itself. To investigate the substrate RNA specificity of ArcS, preQ0-modified tRNA was fragmented using DNAzymes, and lysine transfer was assessed for each fragment (see Fig. 1 above).
Surprisingly, ArcS transferred lysine to all RNA fragments containing preQ0. In the 21-nucleotide (21 nt) RNA fragment, not only the whole tRNA structure, but also the D-arm structure was disrupted. This result demonstrates that ArcS does not recognize the three-dimensional structure of substrate RNA.
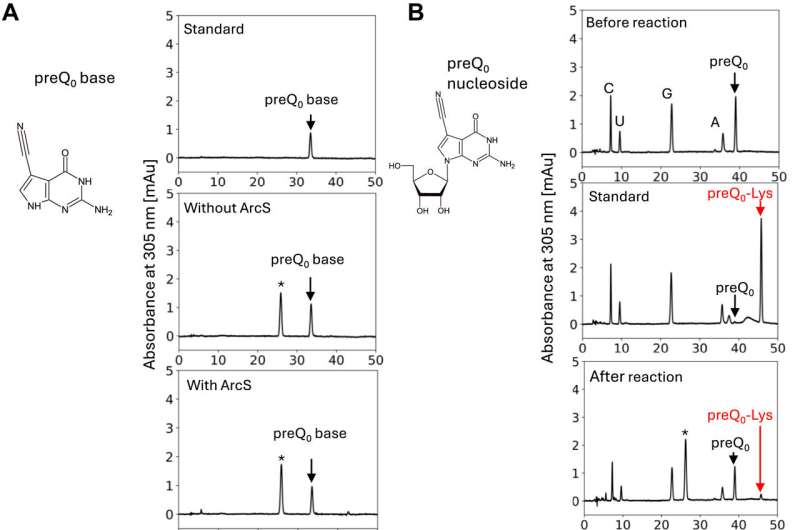
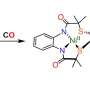
To identify the minimum substrate, lysine-transfer was assessed using the preQ0 base, preQ0 nucleoside, 5'-phosphorylated preQ0 nucleotide, and 3'-phosphorylated preQ0 nucleotide (Fig. 2).
It was found that the minimum substrate was the preQ0 nucleoside, with reaction efficiency increasing when a phosphate group was attached to the 5' position. Thus, ArcS is an unprecedented tRNA modification enzyme that can act on a nucleoside as the substrate.
With the development of mRNA vaccines for COVID-19, modified nucleosides like pseudouridine and 1-methylpseudouridine are effectively used, and research on introducing various modifications into target RNAs is being conducted globally. The discovery of ArcS, which can utilize a nucleoside as a minimum substrate, provides new insights into the synthesis of precursor molecules for these modified nucleosides.
More information: Shu Fujita et al, ArcS from Thermococcus kodakarensis transfers L-lysine to preQ0 nucleoside derivatives as minimum substrate RNAs, Journal of Biological Chemistry (2024). DOI: 10.1016/j.jbc.2024.107505
Journal information: Journal of Biological Chemistry , Nature Chemical Biology
Provided by Ehime University